PCS Clinical Competencies RN
Fact:
All blood components (plasma, cryoprecipitate, red cells, and platelets) must be administration with a 170-260 micron filter. The filter eliminates cellular debris, small clots and fibrin strings which may harm the patient if not removed.
Fallacy:
"Blood components must be ordered directly from Puget Sound Blood Center (PSBC) by phone." This is false! Orders for blood components must be made by telephone to Transfusion Support Services (TSS) (731-3088).
Fact:
When ordering a T&C or T&S, the "Unit/Service Copy" of the "Request for Blood and Blood Components" form should be kept in the patient's chart.
The top page of the "Request for Blood and Blood Components" form and "Hospital Copy" need to be sent with the blood bank specimen to TSS.
Fallacy: All blood components should not be kept out of a monitored blood refrigerator for more than 30 minutes. Platelets or cryoprecipitate should not be placed in a portable refrigerator. Store them at room temperature. Refrigeration damages these types of components. As far as Red cells and plasma, they should not be kept out of monitored refrigeration for more than 30 minutes.
Fact: A RN must accompany a patient off of the patient care area while the patient is receiving a transfusion.
Fallacy: A blood component cannot be transfused if the armband states the patient's middle name but the "Transfusion Report" does not.
The patient's first and last name and medical record number on the "Transfusion Report" must exactly match the corresponding information on the patient's armband. If the "Transfusion Report" is identical to the patient's armband except the middle name/middle initial, it is acceptable to transfuse the component if there is not an obvious discrepancy (e.g., middle name on ID band reads "G" but the "Transfusion report" reads "Gary" is acceptable.)
1. What is required to prevent the specimen from being rejected? The specimen will be rejected if the tube and request form are not clearly labeled (missing, incorrect, or illegible) or do not contain the following:
- The full patient name as it appears on the patient's armband
- Medical record number, including the "H"
- Date drawn (including the year)
- Time drawn
- Signatures of two different clinicians on the requisition and specimen
- Double labeled specimens will result in rejection
2. Can computer generated labels be used for the type and crossmatch /blood bank labels? YES, A computer generated label or a pink "Blood Bank" label is acceptable.
ü There must only be one label on the tube.
ü When an addressograph or computer generated label is not available, the medical record number and the patient's first and last name may be handwritten on the label.
3. Can Medical Assistants that have gone through the phlebotomy training, draw blood bank specimens such as the type and cross or suspected transfusion reaction work-ups? NO, Medical assistants cannot draw blood bank specimens. The phlebotomy training did not consist of this procedure.
ü Two licensed clinicians (Phlebotomists, RNs, ARNPs, PAs, and physicians) may draw blood bank specimens.
4. Does a copy of the "Request for Blood/Blood Components" have to be kept in the patient's chart? YES The green "Unit/Service Copy" of the request should be kept in the patient's chart.
ü The top two pages of the form should be sent with the specimen to TSS.
An acute hemolytic transfusion reaction is the immunologic destruction of transfused red cells, most commonly caused by an incompatibility due to antibodies in the recipient's circulation reacting to foreign antigens on the transfused red cells. The most common cause of an AHTR is the transfusion of ABO-incompatible blood due to patient identification errors occurring anytime during the transfusion process (including obtaining the type and crossmatch specimen). Risk of an AHTR is approximately 1:25,000. Fatalities resulting from an AHTR are approximately 1:160,000.
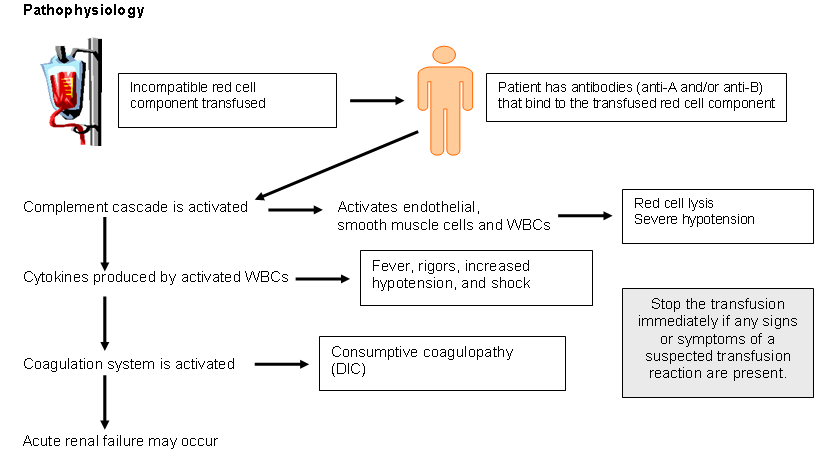
SymptomsSymptoms may include an increased temperature and pulse rate, chills, dyspnea, chest or back pain, abnormal bleeding, and shock. Patients who are anesthetized or sedated may show signs of hypotension and consumptive coagulopathy (DIC).
Planned ActionComplete a pink "Report of Suspected Transfusion Reaction" form (for all suspected transfusion reactions), collect appropriate blood and urine specimens as indicated on the form and send to TSS. If a severe reaction has occurred, send blood component bag and infusion set to TSS.
TreatmentTreatment measures include maintaining arterial blood pressure, correcting coagulopathy, and promoting or maintaining urine output.
Complete a pink "Report of Suspected Transfusion Reaction" form (for all suspected transfusion reactions), collect appropriate blood and urine specimens as indicated on the form and send to TSS. If a severe reaction has occurred, send blood component bag and infusion set to TSS.
TreatmentTreatment measures include maintaining arterial blood pressure, correcting coagulopathy, and promoting or maintaining urine output.
Last modified: 5/23/2006 3:42 PM