EDS
(energy dispersive spectroscopy)
EDS
(energy dispersive spectroscopy)

X-rays are a part of the electromagnetic spectrum and as such have the properties of waves, and of particles of energy. These two properties of the electromagnetic spectrum are inversely related via this simple expression:

E = 12.4 / λ
where
E = energy
λ = wavelength

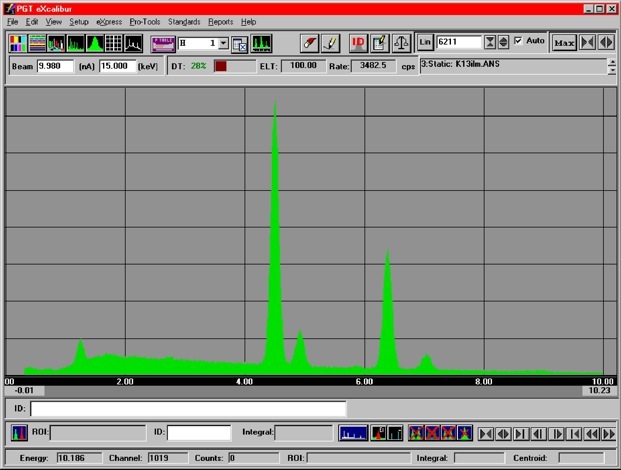
In EDS spectroscopy, x-ray energies are used to identify and quantify the elements present in a sample.
An EDS spectrum of the mineral Ilmenite is shown on the left. The x-axis is Energy (keV), the y-axis is counts. Because x-rays are ‘characteristic’ of the elements they come from, x-rays emitted from Fe will always have the same energy (and wavelength), and fall at the same position in the spectrum, etc. X-rays from all the elements present in the sample appear to be counted simultaneously.
Fe
Mg
Ti
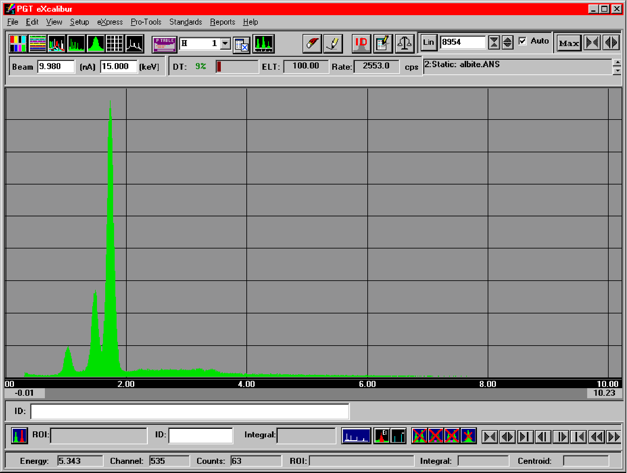
An albite spectrum is shown at the left. While these spectra were collected with a 100 seconds counting time, mineral identification requires only a few seconds of counting time. In an EDS spectrum, x-ray lines for all the elements with concentration greater then about 2000 ppm can be seen.
Na
Al
Si
In addition to the rather high detection limit of about 2000 ppm, EDS suffers from having poor spectral resolution; meaning x-ray lines from different elements may not be resolvable if their energies are similar. For example, Sr overlaps Si and Ba overlaps Ti. WDS has much better spectral resolution. EDS though, is much faster.
